ISO 14155:2020 update released!
Clinical investigation of medical devices for human subjects — Good clinical practice (GCP for Medical Devices) has undergone a significant review. The changes include; — inclusion of a summary section of GCP principles — reference to registration of the clinical investigation in a publicly accessible database — inclusion of clinical quality management — inclusion of […]
Novotech Releases Latest Snapshot of Australian Clinical Trials Activity
The latest Novotech snapshot of Australian #clinicaltrial activity has been released. It indicates there’s upwards of an 80% return to business across some of our highest performing sites. Other key items to note are a broadly accepted use of #eSignature, a reduced impact on #IMP dispensing,and improved #HREC and #RGO timelines. Does this reflect your […]
Global Science v Global Equity: the challenges of testing #Covid19 vaccines.
To rapidly and effectively test vaccines, they must be tested in regions where viral spread and case numbers are high. With Covid19, high incidence has been in groups or countries already marginalised or economically disadvantaged. If testing in these regions is necessary, then it is critical that protections for the vulnerable are robust and that […]
COVID-19, Clinical Trials and the Life Sciences – Understanding cyber and liability risks of the virtual and digital world: Webinar Recording

Another great session conducted as part of PRAXIS’ ongoing webinar series. A big thank-you to our sponsors Newline and Lockton Companies Australia for their support.
The Need for Greater Investment in Australian R and D
The call for greater investment in Australia’s R&D sector has again been made. Perhaps it’s never been more important than now, as our biotech industry faces significant hits, largely not supported by govt relief funding efforts, and our Universities seek ways to support the continuation of research programs in the face of huge economic impacts […]
COVID Clinical Trials: Investigation for Investigation’s Sake?

In the rush to find a treatment for #Covid19, we’ve seen a huge influx of new #clinicaltrials, largely of repurposed drugs. Many of these trials are small and unlikely to prove or disprove efficacy in a significantly meaningful way. Yet, studies are approved, participants are enrolled and resources wasted, as for some players, the cash […]
The 6 New Principles of Animal Research
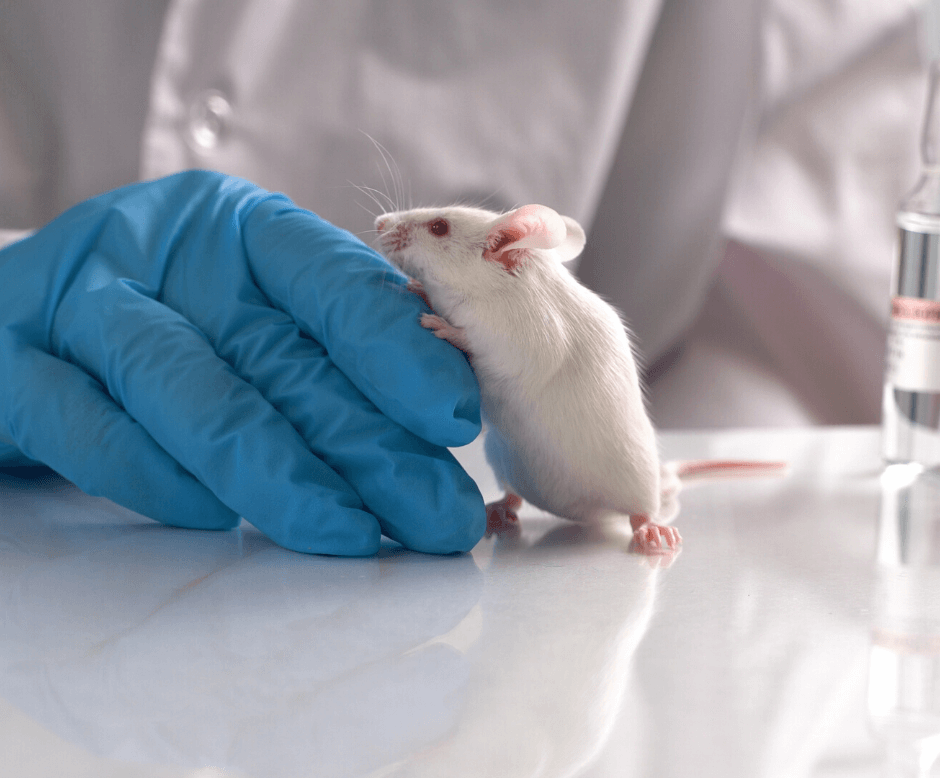
6 new principles to replace the 3 R’s (replace,reduce,refine) in animal research. The new principles ask researchers to think more critically about their research and the ethical basis for its conduct. #animalresearchethics #researchethics #research Full article
PRAXIS’ Commitment to Aboriginal and Torres Strait Islander Communities

Statement of Support PRAXIS Australia is committed to supporting our Aboriginal and Torres Strait Islander friends, colleagues, and communities across Australia, and stand proudly alongside, as we together recognise the harms done over the last 232 years. As an organisation dedicated to excellence in research and research ethics, we recognise that we must be inclusive […]
Red Flags and Whistle Blowers: an incredulous case of Clinical Trials fraud

This story depicts an extended case of deliberate fraud and misconduct by an Investigator against a list of CROs and Pharmaceutical companies. Was the need for rapid start up the catalyst that resulted in this chain of events that impacted patient safety, breached all research ethics principles and corrupted drug development? Via Carol Bognar #clinicaltrials […]
The Perils of Impatience and the Risk of Rapid Research

You may be aware of the much discussed retraction last week of the #Lancet article which claimed that hydroxychloroquine was associated with increased in-hospital mortality and ventricular arrhythmia when used to treat #Covid19. The harms caused by this publication are many. Importantly, it causes the public to question their faith in #medicine, #research and #clinicaltrials […]
A guide to Social Media and #ClinicalTrials #ICTD2020

In our PRAXIS Australia Ltd webinar today we talked about the growing role and importance of social media and artificial intelligence to support #clinicaltrialrecruitment and retention. Opyl Ltd and Association of Australian Medical Research Institutes (AAMRI) have recently released a guide to help you understand some of the fundamental elements – for those new to […]
International Clinical Trials Day Research Essentials Discount Offer
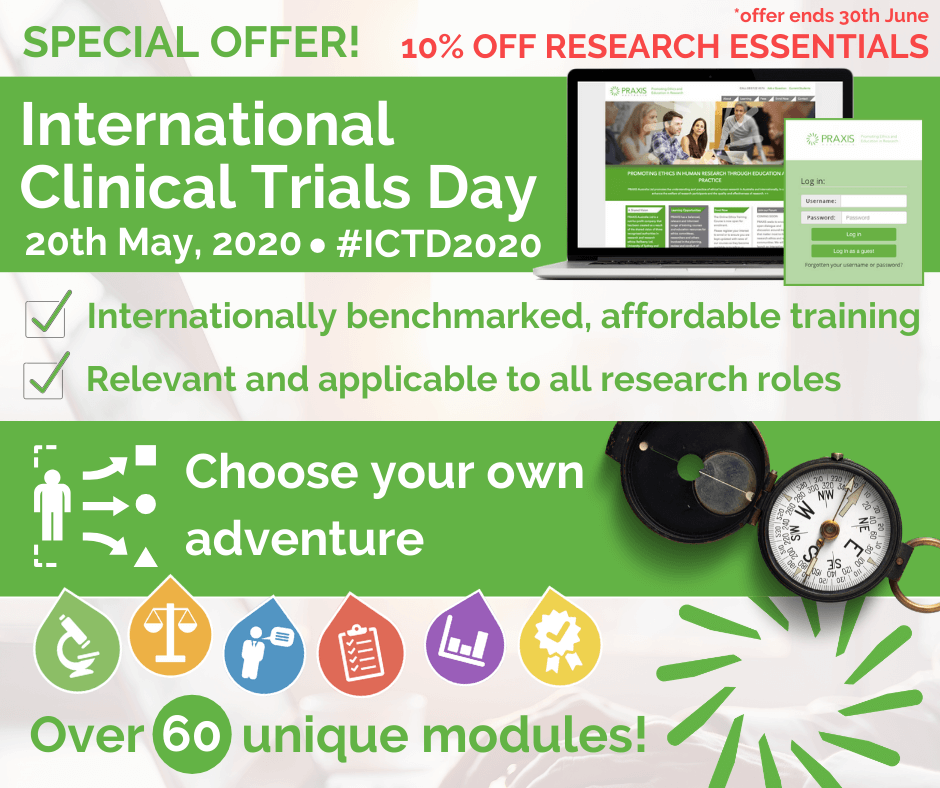
Happy International Clinical Trials Day 2020! Today we celebrate the many ways #clinicaltrials have improved health outcomes for individuals and communities, and the people who make it possible, across every aspect of the sector. Thank you. In recognition of our workforce, we are offering a 10% discount on our online #ResearchEssentials course till end June. Full course: […]